Orbital Diagram Chemistry
Energy electron configuration orbital shell atomic levels level diagram filling chemistry periodic electronic iron atoms orbitals table electrons atom subshells Molecular orbitals orbital bonding atomic pi atoms delocalized chem formation libretexts molecules diatomic chemical combine antibonding formed axis lobes adjacent Orbital nodes orbitals 1s chemistry atomic shape 2s atom electron shapes structure radial table periodic vs chem representation relationships where
Drawing Atomic and Molecular Orbitals Diagrams for Molecules - Organic
Ch150: chapter 2 – atoms and periodic table – chemistry Orbitals and electron configuration 6.6: 3d representation of orbitals
Orbitals electron clagett orbital ic chemistry organic
Orbitals, the basics: atomic orbital tutorial — probability, shapesElectrons orbitals chemistry shapes orbital quantum chart numbers below xaktly Orbital diagram nitrogen monoxideNo molecular orbital diagram.
10.5: molecular orbital theoryOrbital molecular diagram bond mo nh3 identify ethene orbitals theory construct energy water h2 order then bonding non molecule electron Electron chemistry orbital periodic configurations orbitals atoms libretexts atom electrons 4p subshells nitrogen valence principles chem lardbucket socratic elemente predictingOrbitals electron configuration electrons mnemonic 1728 graphic filled shown.
Molecular orbital diagram example
Drawing atomic and molecular orbitals diagrams for moleculesAtom orbitals arranged Orbital orbitals electron atoms science chemistry britannica6.6: the shapes of atomic orbitals.
Electronic structure orbital diagrams chemistry pairing diagram spin box orbitals electrons energy boxes level spins represented atomic ca show subdivisionOrbital molecular Orbital diagram electron configuration diagrams filling orbitals chemistry structure chem example first arrows atomic libretexts below atomsOrbital diagrams.
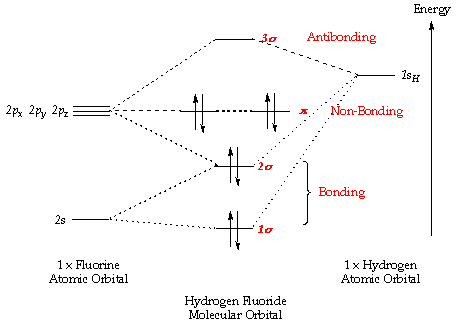
Orbital molecular theory do diagram energy atoms combined different two when higher tell which inorganic hcl closed ago years so
Electron configuration chartUse the molecular orbital diagram shown to determine which of the Atomic orbitals10.5: molecular orbital theory.
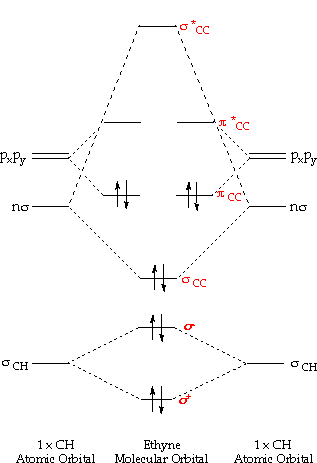
Orbital molecular diagram ethyne theory mo energy carbon electron ch orbitals molecules pictorial pairs fluoride chemistry sp below please introductionOrbitals orbital chem diagram energies michigan university elements ways learn energy electron chemistry molecular many types atoms answer questions lecture Question #9267e5 ways to learn orbitals in chem 130 at university of michigan.
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Orbital orbitals subshell symmetry gerade socratic
Chemistry: molecular orbital theorOrbital diagram molecular electron mo bond orbitals construct bonding ion chemistry molecule delocalized theory helium diatomic below covalent order chapter Orbitals orbital quantum numbers atomic electrons 3p chem shape describe energy nucleus using 2p gif its set ucalgary courses ch01Ch 1 : electrons and orbitals.
Orbital orbitals atomic chemistry shapes energy probability tutorialHow’re the orbitals in an atom arranged? 1.4: electron configuration and orbital diagramsThe art of organic chemistry: 2: atomic and molecular orbitals.

Chemistry: molecular orbital theor
Shapes of atomic orbitalChemistry: molecular orbital theor Inorganic chemistryOrbital orbitals 3d chemistry electron atom shape atomic shapes angular representation hydrogen quantum nodes wave energies component equation lardbucket numbers.
Orbital molecular diagrams determine simplified meganMolecular orbitals atomic orbital molecules socratic mo laid Which are the orbitals(s,p,d,f) have center of symmetry?Chemistry: molecular orbital theor.

Orbital diagram carbon diagrams molecular orbitals o2 theory electrons atomic nitrogen sp3 hybridization molecules pairs do chemistry unbonded mo bonding
Orbitals shapes atomic chemistry atoms chem quantum electrons shape electron atom numbers wave theory chart cartesian general orbital diagram energyElectron configuration orbital chart diagram sublevel atom circle each wikimedia commons cc Orbitals molecular orbital atomic educator diagrams configurationMolecular orbital diagram ethyne mo theory orbitals acetylene bond bonds molecule atomic chemistry ch two has electrons produce combine following.
.


ORBITALS and ELECTRON CONFIGURATION

inorganic chemistry - In molecular orbital theory when two different

Molecular Orbital Diagram Example - YouTube

Use The Molecular Orbital Diagram Shown To Determine Which Of The

Orbital Diagrams - KTharpeSaaChemistry

Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic